Name ________________________ Net ID_______________
TA Name____________________ Discussion Section________
Physics 212 James Scholar Assignment #2
Due:
The
problems are to be done on paper,
showing all work. Again, the
presentation should be neat, legible, and easy to follow. {Remember to include your
name, netID, and Discussion section and ideally TA name on the top of the page!
Please turn in your assignment into the Grading Box labeled “Physics 212 James
Scholars”, located on the 2nd floor, in the interpass between Loomis
and MRL (Materials Research Lab). The interpass is down the hall about 10 meters east
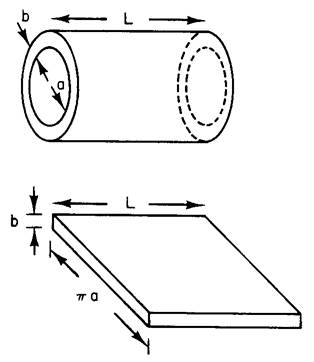
of Room 262 Loomis, where half of you have your Lab.} Part I A simple model of a nerve cell (axon) is a hollow
cylinder of length L, inner diameter “a”, and membrane thickness “b” (left
figure). The membrane, which is similar in structural and electrical properties
to olive oil, is a dielectric, which electrically (and structurally) separates
the inside and outside of the axon. (a) Treat the cell as a hollow cylinder of length
L = 1 cm, a = 2 μm (2 microns), b = 5 nm, and a dielectric constant of the
membrane , κ = 3. What is the capacitance C? Calculate this two ways: i. Calculate it correctly, for the cylindrical
geometry shown above. ii. Approximate it
as a parallel plate capacitor, by “cutting” the axon open and laying it flat,
as shown in the bottom sketch. [Note:
the longest nerve cell in your body, the Sciatic nerve, runs from your lower
spine to your foot, roughly 2 ½ - 3 feet in length!] In experiments on nerve axon (and muscle fibers too)
cells two electrodes are inserted into the cell (axon) close to each
other. One electrode serves as a signal
generator of rectangular pulses. The
other serves as a monitor/detector of the cell response to the signal
pulses. The electrical characteristics
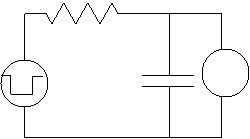
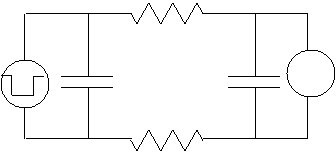
of the cell in such an experiment may be represented by the equivalent circuit
illustrated in the left figure, where C
is the capacitance of the cell membrane, R
is the resistance of the cell membrane, the left voltage source is the signal
electrode, and the voltmeter (on the right) is the receiver electrode. Note
that since the left capacitor will basically charge almost instantly (the
voltage across it equals the voltage from the generator), we can simplify the
circuit to that on the right.![]()
(b) Use the response
graph below to estimate the time constant of the cell.
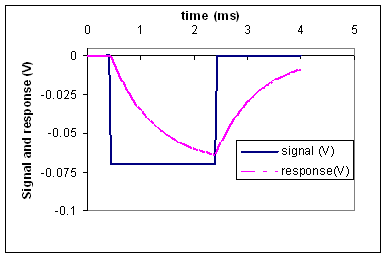
(c) Using the
result of parts (a) and (b), calculate the value of the resistance, R. Note: this is the resistance of the
membrane to the flow of ions. You can now see how effective an oily-membrane is
in preventing ion flow – this is essential to life because the ion
concentration inside a cell and outside a cell is very different. Furthermore,
life uses the energy stored in the capacitance of cells to do all sorts of
things, including making “action potentials” in nerves (which is you thinking)
and making a high-energy compound called ATP (which is the “food” of the cell).
If the potential difference (voltage) across the
membrane is 70mV (a typical value), find:
(d) the electric field through the membrane. Is this a relatively small or large value? Compare to dielectric strength of air, approximately 3 million volts/m. Is the dielectric strength of the membrane greater than or less than that of air?
(e) the charge on each side of the membrane (to
account for this typical potential across the cell membrane).
(f) This charge is caused by a slight imbalance
between the number of positive ions (either K+ and/or Na+)
and the number of negative ions (primarily Cl- ions) inside the
cell. First assume there were no charge
imbalance at all. If the cell has 150 mM
KCl inside (a physiologically reasonable number; “mM” = millimolar = 10-3
moles/liter), calculate the total number of K’s (which is then also the total number
of Cl’s) inside one cell. Next from (e)
calculate how many more K+ than Cl- there actually are in
the cell.
(g) Express your results from (f) as a fractional
imbalance, i.e., (#K+ - #Cl-)/#KCl.
All of our nerve action – our ability to think, feel,
hear, see, move…everything!… relies on this exquisite control of ion
(electrical) imbalance.
Part 2
Do a little research, and write a short paragraph
about “iontophoresis”, including a basic description of how it works, and 3
possible applications.