Today
:
Next time
:
Something's Missing
There were two types of problems with classical physics.
First, there was something very major missing, since there was no explanation of any chemical properties, mechanical properties, phase transitions, colors, etc of materials- or even an explanation of why the atom wouldn't collapse. So it looked like some whole new set of force laws or something was needed to describe the world at the scale of atoms and molecules. It might seem that filling in these huge missing pieces, where unknown ingredients were needed to make predictions, was a giant task, but one that could be performed within the confines of classical physics.
Second, and much more serious, there were a small set of problems for which classical physics made predictions that were wrong. We'll follow the track of these problems, because historically it was these sharper problems which led to the new physics.
The black body problem
Thermodynamics and statistical mechanics were two of the success stories of 19th century physics. One could explain properties such as specific heat with a simple statistical law.
Equipartition of energy
. When one has a large collection of objects in thermal equilibrium, the average amount of energy in each "mode" of motion is kT/2. (k is Boltzman’s constant, T is absolute temperature)A mode of motion is an independent motion. For example, motion of each molecule along x, y, and z are three modes. Rotation and vibration also, depending on molecular structure.
What about the thermodynamics of waves (e.g., light)? We know that hot objects emit light, so we must understand it.
Consider waves on a string (or light in a mirrored box). The modes consist of the various standing waves:
![]()
There are an ° number of modes, mostly at very short wavelengths (high frequency). This seems to imply that
there should be infinite energy in the EM radiation at any finite T. We would all be glowing infinitely brightly! This is called the ultraviolet catastrophe, because the infinite amount of energy appears in the high-frequency (ultraviolet and higher) modes.
Equipartition worked up to some frequency (which depends on T) but not at higher frequencies
Planck proposed, in 1900, to modify the law describing the interaction of radiation with matter. He suggested that
energy can only be emitted or absorbed in integral multiples of hf. That is, 0, hf, 2hf, 3hf, etc. are allowed, but not 0.5hf.This has the effect of suppressing the high frequency modes, because they require so much energy that they are not excited at all most of the time. (An exponential suppression follows directly from the laws of stat. mech., just as equipartition follows if continuous values of the energy are possible.
Planck’s hypothesis gave the right answer, but had no physical motivation.
For example, is the phenomenon a property of the light, the atoms, or of the interaction between them? Is this an epicycle? It breaks the seamless description of motion and energy just as surely as an epicycle would break a crystal sphere.
Heat Capacity of Solids
The energy density in solids goes up as T is increased, and the heat capacity is a measure of how much energy must be added per degree change of T. The heat capacities of solids at temperatures of around room temperature or higher are usually in agreement with equipartition, but at lower T the heat capacities become very small.
Debye (1912), following a cruder idea of Einstein (1907), showed that this behavior would result if:
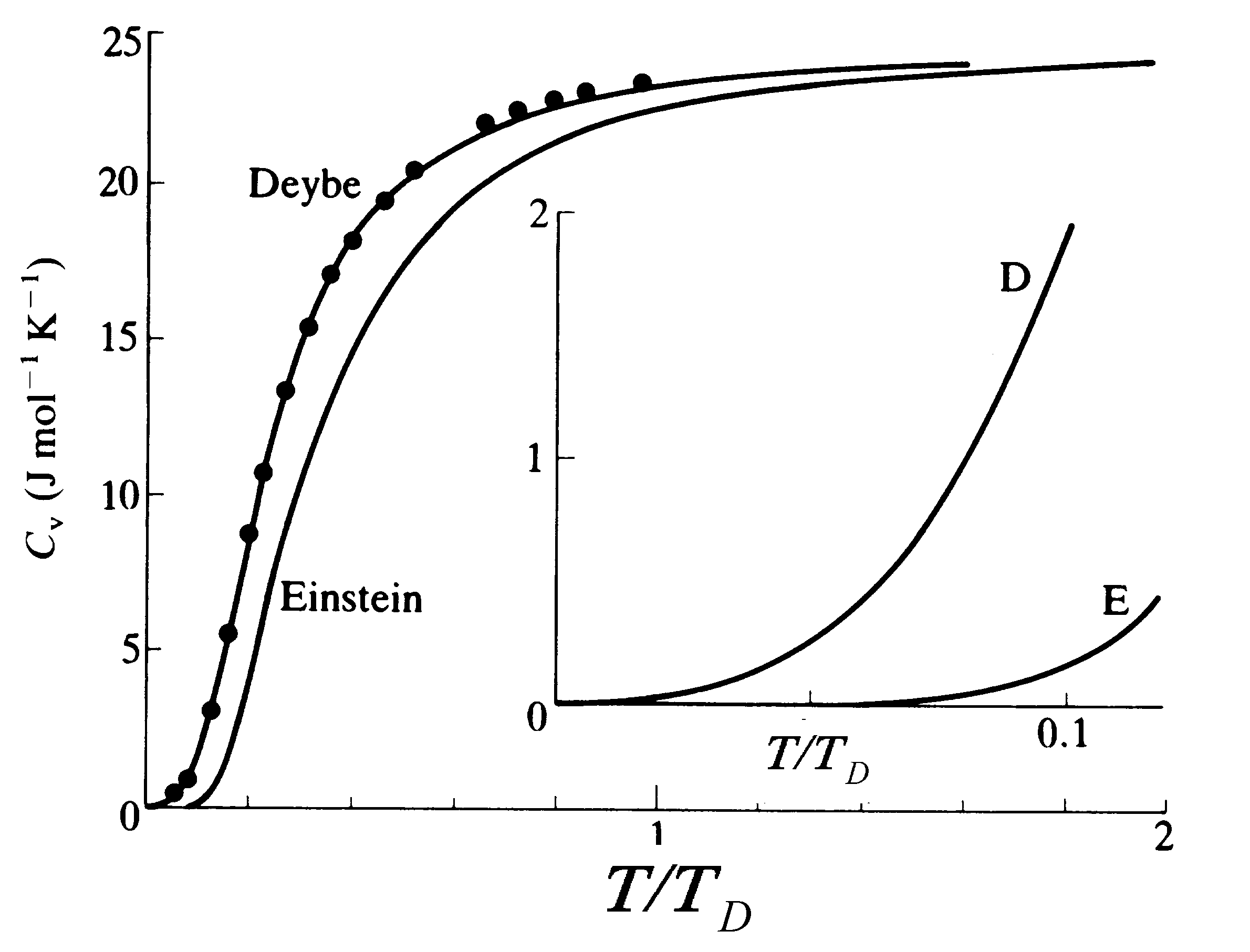 The data points here
The data points here
are for silver.
Photoelectric effect
Hertz discovered in 1887 that shining UV light on metal electrodes can induce sparks across a voltage gap. Red light, no matter how intense, can’t do it.
In 1905, Einstein proposed that Planck’s solution to the BB spectrum problem could, if extended, explain this effect. He suggested that
the quantization of the EM energy was not in the interactions with matter, but a property of the radiation itself. That is, light waves come in little packages, or quanta (photons), each of which has a specific amount of energy, hf.This hypothesis led to several predictions about the behavior of the photoelectric effect. If the electrons in a metal are bound to it by a certain amount of energy (call it E
o), then:In the wave picture of light, one expects no significant frequency dependence, only an intensity dependence. That is, the only important quantity is the rate at which energy is put into the metal.
Einstein was verified by Millikan in 1914.
How can waves behave like particles?
Compton effect
If one shines a light
wave on a free electron, the electron will oscillate in response to the electric field. This will cause the electron to emit radiation with the same frequency as the incident light.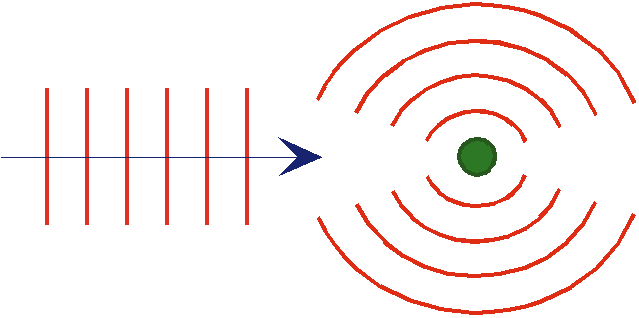
What actually happens? The emitted radiation has a frequency corresponding to the energy light would have if it were a
particle of E = hf colliding with the electron.
The
energy of the scattered particle (frequency of the light) depends on the angle. This effect is only sizable when hf ~ mc2, so the "light" needs to be gamma rays. This is due to SR kinematics, not any special property of light.This effect was first observed in 1923 and confirmed the view that in some circumstances light behaves like a particle.
Atomic spectra
Atoms and molecules are observed to emit specific wavelengths of light. One can identify atoms and molecules by looking at the spectra. This phenomenon cannot be understood easily in classical E&M. The frequency of emitted radiation depends on the frequency of motion of the electric charges, and it is hard to see why the motion should be restricted like that.
In hydrogen, the frequency spectrum follows a simple pattern:
f = const * (1/n2 - 1/m2) (Ritz)
where n and m are integers.
With the discovery of the electron by Thomson in 1897, the question became,
what is the structure of the atom?In 1910, Rutherford showed that the atom’s positive charge is very heavy and also very small. Are the electrons orbiting the nucleus like the planets orbit the Sun?
This is an appealing picture, but it has
a fatal flaw. As the electrons orbit, they should emit radiation and lose energy. They will spiral into the nucleus in about a nanosecond. Needless to say, this is not how atoms behave.The planetary atom also does not explain the discrete spectrum, since orbits can have any frequency.
The Bohr atom:
a suggestive temporary ad-hoc fixIn 1913, Niels Bohr postulated that quantization applies not only to photon energy, but also to the electrons in their atomic orbits. He proposed that
the orbital angular momentum of the electrons could only take on discrete values, namely integral multiples of Planck’s constant divided by 2¹ (L = nh/2¹).
Not to scale. The radii of the orbits
are proportional to n2.
This proposal "solved" both of the problems.
This is a partial solution, but it leaves much unexplained. For example, if only certain orbits are allowed, how does the electron get from one to another? Also, why is the angular momentum quantized? How is this connected with the quantization of light?
It is
an amazing coincidence that Planck’s constant describes both electrons and light (as well as sound) ..., and although the Bohr model was wrong in all of its essentials, it was extremely important for demonstrating that Planck's constant had SOMETHING important to do with atomic structure.
Electron diffraction
Davisson performed a key series of experiments
(1921-7).He scattered electrons from crystals and showed that they preferred to bounce in particular directions. These directions were exactly those which one would expect if
electrons are waves of wavelength l = h/p.
This is the same behavior that X-rays show, and was the evidence that had been used 30 years before to show that X-rays are waves (part of the EM spectrum).
How can particles behave like waves?
We are in an odd situation.
Light, which is usually seems to be a wave, seems to exhibit particle properties. Electrons, which usually seem to be particles, sometimes exhibit wave properties. Planck’s constant is the common connection between the phenomena.
Note: E = hf and p = h
/ l are just two manifestations of the same relationship. The 4-d nature of spacetime tells us that energy and time are related in the same way as momentum and space. Otherwise, the Lorentz transformation would fail.
The relation p = h
/ l was first proposed on this theoretical basis by A. C. Lunn (U. Chicago) in 1921, and subsequently by L. deBroglie (1923). Lunn's paper was not accepted by the Physical Review, so p = h / l is known as the deBroglie relation. ( Davisson had been a student of Lunn, who urged his students to explore the "wave properties of beta radiation".)
Our old particles turn out to be waves, and our old waves
turn out to be particles!
the "particles" have frequency, wavelength…
the "waves" have lumps of energy, momentum….
The old dualism (world consists of particles interacting by continuous fields) is gone- everything consists of quantum objects which have both wave-like and particle-like aspects, which become relevant in different situations.
We'll see that
the common claim that these objects are both waves and particles is false- they're just something else, with a resemblance to both classical waves and classical particles, but also with properties of neither.Notice that at first glance we seem to be saying something very incoherent. A wave cannot have a wavelength, even approximately, unless it is spread out over distances large compared with the wavelength. A particle is supposed to have a particular position. How can we say "the momentum of the particle is given by its wavelength?"
De Broglie’s hypothesis
De Broglie proposed that
every particle has an associated wave (called a pilot wave), and every wave has an associated particle. The relationship between the two is always the same:E = hf
and p = h/l (or vector version)How does this solve the puzzle of the Bohr atom? It doesn't yet- but there's a suggestive relation: if there are an integer number of DeBroglie wavelengths around a circular orbit, Bohr quantization results!
The full solution requires understanding what persistent wave patterns can exist in the atom, which requires finding the wave equation. The waves will be genuine 3-D waves, not waves on an imaginary 1-D orbit.
The electron is described by a
wave function, y(r,t).This wave function obeys a differential equation called Schrödinger’s equation. (also probably initially due to Lunn)
![]()
The first term depends on how
y wiggles in space, which gives the momentum. The second term gives the potential energy, due to various neighbors. The third term gives the energy, which is how fast y changes in time.