


Next: Project Goals
Up: Introduction
Previous: Parallel Tempering
Water, although abundant and apparently simple, is a model complex liquid, making simulation very challenging. In a crystal, an 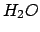 molecule will coordinate tetrahedrally with its nearest neighbors due to the relatively strong interaction between the nearly bare protons and the highly electronegative oxygen nucleus. In the liquid, thermal excitations break these hydrogen bonds, resulting in a disordered extended random network of moleucles whose energetic preference for tetrahedral order is frustrated. Orientational degrees of freedom are constrained by interactions among the strongly polar molecules, with the result that the system can persist in a given (arbitrary) random network for a very long time, not effectively exploring other configurations that could be energetically more favorable. Thus, liquid water is a good candidate for the application of PT.
molecule will coordinate tetrahedrally with its nearest neighbors due to the relatively strong interaction between the nearly bare protons and the highly electronegative oxygen nucleus. In the liquid, thermal excitations break these hydrogen bonds, resulting in a disordered extended random network of moleucles whose energetic preference for tetrahedral order is frustrated. Orientational degrees of freedom are constrained by interactions among the strongly polar molecules, with the result that the system can persist in a given (arbitrary) random network for a very long time, not effectively exploring other configurations that could be energetically more favorable. Thus, liquid water is a good candidate for the application of PT.
Figure 3:
While the solid phase of water (ice) is tetrahedrally ordered (left), the liquid (right) consists of a disordered random hydrogen bond network; the system is frustrated and glassy. Ice image from http://www.its.caltech.edu/ atomic/snowcrystals/primer/primer.htm.
![\includegraphics[width = 0.5\textwidth]{data/images/ice.eps}](img15.png)
![\includegraphics[width = 0.5\textwidth]{data/images/ST2_1.eps}](img2.png) |
John Gergely
2006-05-12
![\includegraphics[width = 0.5\textwidth]{data/images/ice.eps}](img15.png)
![\includegraphics[width = 0.5\textwidth]{data/images/ST2_1.eps}](img2.png)
![\includegraphics[width = 0.5\textwidth]{data/images/ice.eps}](img15.png)
![\includegraphics[width = 0.5\textwidth]{data/images/ST2_1.eps}](img2.png)