Nuclear fusion is the process of combining of light elements into
heavier elements (the opposite of nuclear fission which is used
to generate electricity in nuclear power plants). Both fission
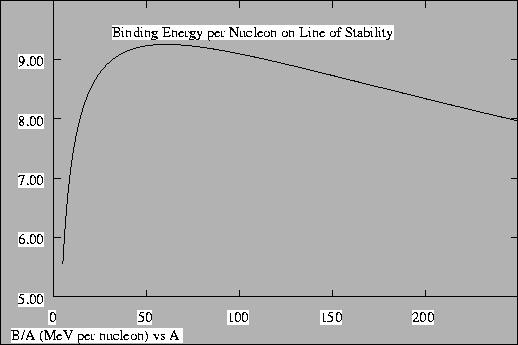
and fusion give off large amounts of energy. This is due to the
binding energy per nucleon, which is small for light ions, peaks
around mass number 60, and decreases again for heavier elements.

Fusion is the energy source which powers the
stars, like our sun. In a star, the fuels for fusion (mainly
isotopes of hydrogen) are held together at high temperature and
density by the gravitational force due to the star's huge mass.
Unfortunately, achieving fusion in a laboratory on Earth is much
more difficult. The problem comes from the fact that in order to
overcome the repulsive electrical force between two positively
charged nuclei, the nuclei must have very high energies. This
translates into extemely high temperatures (millions of degrees
Celsius), at which the hydrogen gas exists as a plasma. A plasma
is a superheated gas, so hot that electrons are stripped from the
nuclei, resulting in a quasi-neutral mixture of ions and
electrons. Confining this plasma at such high temperatures poses
an extremely challenging technological challenge and has been the
goal of fusion research for decades.
If fusion is so difficult to harness, one may wonder why
scientists are spending so much time trying. The reason is that
if nuclear fusion can become a viable energy source (e.g. for the
production of electricity) it would very likely be the ultimate
energy source - the solution to all of our energy problems. This
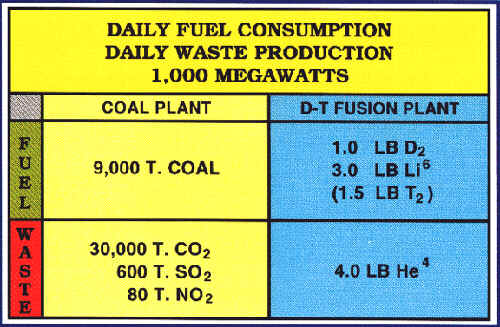
is because the fuels for fusion are abundant. Hydrogen isotopes
like deuterium are found in the seawater that covers most of the
Earth. Tritium, another isotope of hydrogen, can be made from
lithium, a common metal on earth. Fusion energy would also be
inherently safe. There is no risk of the nuclear accidents that
plague fission power, no high level nuclear waste is produced,
and there is no generation of weapons material like the plutonium
from fission reactors. Also, fusion energy would be exceptionally
clean - producing no air pollution. Today's fossil fuel powered
power plants are the worst polluters and pose a serious threat to
the enviornment.
The most promising technique for harnessing
fusion power on Earth is the use of strong magnetic fields to
confine the fuel ions. The reactor would most likely be in the
shape of a torus, or donut shape. The easiest fuel to use seems
to be a mixture of deuterium and tritium. This reaction has the
largest fusion cross section of any combination of fusionable
materials. The reaction is ![]()
|
 |
One type of fusion reactor called a Tokamak (a Russian language acronym for "Toriodal Magnetic Chamber") utilizes strong magnetic fields, on the order of a few Teslas, to confine the fuel. Examples of reactors of this type are the Tokamak Fusion Test Reactor, which is no longer in operation, and the Joint European Torus. Energy is usually obtained in these reactors via the D-T reaction mentioned above. For a typical 1 GW reactor design, there are about 0.6 GW of power going to ions (the alpha particles). The neutrons, which carry even more energy from the fusion reaction, pass through the walls more easily and actually deposit their energy outside the first wall. However, in steady state, the 600 MW from alphas must be absorbed by the walls. For a reactor of this size, the surface area of the walls would be ~600 m^2, leading to an average power deposition to the walls of ~1 MW/m^2. However, in certain locations, the power deposition will be locally much higher due to the configuration of the reactors. Below is a cross section of a typical Tokamak design. The field lines outside the seperatrix do not close on themselves but rather are "open", intersecting some material boundary. This wall area, called a divertor, is where the peak power loads occur and hence the choice of materials here is crucial. Graphite tiles are often used as divertor materials due to their good thermomechanical properties. Graphite can handle these large heat loads without its stucture being degraded. However, because the plasma is composed of hydrogen and because of the reactivity between carbon and hydrogen, the graphite is susceptable to a process called chemical sputtering whereby carbon is lost from the surface in the form of hydrocarbon molecules, mainly methane (CH4). A critical question is then whether the hydrocarbons are lost to the core plasma or if they are locally redeposited on the wall. While the gross erosion rate may be high, if the redeposition fraction is high, net erosion can be kept low. It is the net erosion which determines the lifetime of wall components and the pollution of the core plasma with impurities.

A field of research which is currently very
important is studying the transport of these impurities to see
whether they are redeposited or lost. These web pages explain a
Monte Carlo code which was developed to do simulate the transport
of hydrocarbons in fusion plasmas. The theory section explains
the theory of impurity transport used in the code. The next
section discusses the implementation of this theory in our code
and gives a link to the Monte Carlo code itself. We also show
some results obtained with the code.