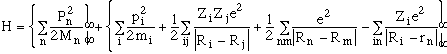
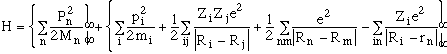
where the kinetic energies and potential energies are apparent. In principle, one should solve the Schrodinger or Dirac equations (e.g., Hy(rn,Ri) = Ey(rn,Ri)) for the system wavefunction y(rn, Ri) and we have an exact solution. Note that, in general, ri are generalized coordinates which may be the position of the atoms or for rigid-rotating molecules, for example, they would be center-of-mass coordinates and molecular orientation, and so on. The Kinetic energy usually has the form of pi2/2mi. (see A&T pg. 6 section 1.3)
Because the mass of the electron relative to the nuclei is small,
or M/m= 100-1000, and the respective frequencies (inverse of the time,
t) of response are
we/wnucl
= tnucl/t
e= M/m, Born and Oppenheimer in 1923 suggested that one could
represent the wavefunction as a product, i.e. y
(rn,Ri)=
X(Ri)f(rn;
Ri). In effect, this just says that the nuclei
move much more slowly than the electrons (and are more or less
fixed as the electrons equilibriate). Notice that the electron
wavefunction depends on the position of the nuclei parameterically.
As a result of this product wavefunction, the problem breaks up
into the separate equations to solve.
The electrons equations is Hel
f(rn; Ri)=
V(Ri)f(rn;
Ri). The equation for the atomic nuclei (using
the effective interatomic potentials V(Ri) which
is given in the second set of brackets above) is given by
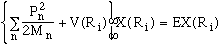 ,
,