


Next: Conclusion
Up: Title
Previous: The setup of the Simulation
Results
During the simulations among other observables the temperature, the pressure, the
kinetic and potential energy as well as the positions have been saved to disk several
times. Click here for an animation of the run. These files were used to analyze the development of the system with time where
the Stillinger criterion [7] was used to determine if a cluster has formed.
In fig. 5 we plotted how the number of clusters, i.e. non-monomers,
changes with time. One can see that the number of clusters sharply rises
immediately after the simulation has been started. After that it falls continuously
until it approaches one.
Figure 5:
the number of clusters as a function of time
|
|
The free energy for the formation of a cluster 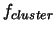 is given by
is given by
where 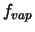 and
and 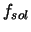 stand for the free energy of the vapor phase and the
solid state, respectively. The term
stand for the free energy of the vapor phase and the
solid state, respectively. The term 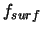 represents the free energy
of the surface which is introduced into the system. Accordingly one can explain
the behavior of the system as follows: under the conditions of this simulation
the vapor is no longer the most stable phase. Instead the system evolves into
the solid state by forming clusters. But because in the beginning there are
many small clusters the surface energy plays governing role. As time
advances the smaller clusters merge to former bigger clusters and in the end
only one big cluster remains. This final cluster is shown in fig. 6
and it can be seen that it has a crystalline structure.
represents the free energy
of the surface which is introduced into the system. Accordingly one can explain
the behavior of the system as follows: under the conditions of this simulation
the vapor is no longer the most stable phase. Instead the system evolves into
the solid state by forming clusters. But because in the beginning there are
many small clusters the surface energy plays governing role. As time
advances the smaller clusters merge to former bigger clusters and in the end
only one big cluster remains. This final cluster is shown in fig. 6
and it can be seen that it has a crystalline structure.
Figure 6:
the cluster remaining at the end of the simulation run
|
|
As described in the preceding section only the Ar atoms are used to control
the temperature of the system. Equivalent to the experiment they act as the cooling
gas for the Cu atoms. The energy which is released during the formation of
clusters raises the temperature of the Cu atoms; fig. 7 shows the
temperature1 of the Cu atoms as a function of time. It can be seen that
it takes some time until the temperature is lowered due to collisions with the
Ar atoms.
Figure 7:
the temperature of the Cu atoms (cf. footnote
1) as a function of time
|
|
Figure 8:
the potential
energy per Cu atom as a function of time
|
|
A snapshot of the system after 100ps is shown in fig. 9 and fig. 10. The clustering
has already begun and several bigger clusters can be identified.
Figure 9:
snapshot of the system at 100ps, showing Ar (blue) as well
as Cu (red) atoms
Click the picture for animation
|
|
Figure 10:
snapshot of the system at 100ps, showing only Cu atoms
Click the picture for animation
|
|
For the same instant of time the cluster size distribution (fig. 11)
and the radial distribution (fig. 12) function have been computed.
Figure 11:
the cluster size distribution (left) and the radial
distribution function (right) after 100ps
|
|
Figure 12:
the cluster size distribution (left) and the radial
distribution function (right) after 100ps
|
|



Next: Conclusion
Up: Title
Previous: The setup of the Simulation
![\includegraphics [width=0.95\columnwidth]{eps/nclustertime2.eps}](img83.gif)
![]() is given by
is given by
![\includegraphics [width=0.95\columnwidth]{eps/sizes2.eps}](img94.gif)
![\includegraphics [width=0.95\columnwidth]{eps/raddist2.eps}](img95.gif)