Project Report:
Introduction
Water Potential
MD simulations
Results
Conclusions
References
Team 2
Xiaohai Li
Vamsi Akkineni
Jianwei Wang
Fall, 2002
Xiaohai Li, Vamsi Akkineni,
Jianwei Wang
Potential[7]
Interactions between
water molecules are far more complicated than those between
particles of simple liquid. This complexity arises from the
ability of water molecules to form hydrogen bonds, making water
an associated liquid. Also it arises from the existence of substantial
non-additive three- and high-body terms which leads to the failure
of the ordinary pair-additivity approximation used in computer
simulation. A lot of studies have shown that the results
of the simulation are highly sensitive to the intermolecular potential
function used.
The intermolecular potential of water molecules used in computer simulation can be grouped into two classes as far as their origin is concerned: empirical and quantum mechanical potentials. In the first case, all parameters of a model are adjusted to fit experimental data for water from different sources, and thus necessarily incorporate effects of many-body interactions in some implicit average way. The second class of potentials obtained from ab initio quantum mechanical calculations represent purely the pair energy of the water dimmer and they do not take into account any many-body effects. But they are usually computationally extremely demanding. So here we focus on the first class of intermolecular potentials.
One of the most widely used intermolecular potentials is ST2 model. It is a 5-point model with four charges arranged tetrahedrally around the oxygen atom (Fig 2c). The positive charges are located at the hydrogen atoms at a distance of 1Å from the oxygen atom, nearly the real distance in the water molecular. The negative charge are located at the other two vertices of the tetrahedron (sites t1 and t2 in Fig 2c) but at a distance of only 0.8Å from the oxygen. The tetrahedrally arranged point charges make it possible to form the hydrogen bonds in the right direction. The totally interaction energy for a pair of molecules i and j can be written as follows:
which basically is Coulomb interactions between all the charged sites plus the Lennard-Jones interaction between the oxygen atoms.
Another empirical water model often used is the TIP4P model. It differs from the ST2 model in several aspects. The rigid geometry employed is that of the gas phase monomer with an OH distance of 0.9572 Å and an HOH angel of 104.52º. The two negative charges are reduced to a single one at a point M positioned on the bisector of the HOH angle at a distance of 0.15 Å in the direction of the H atoms(Fig 2b). It also can written as a Coulomb term plus a (12-6) Lennard-Jones term.
All these potentials discussed above are
all fixed point charge model. Actually none of them can quantitatively
reproduce thermodynamic and structural properties of water
over a broad range of temperature and pressures. A lot of work
has been done to include additional terms besides the two-body
effective potential, such as molecular polarizability or intramolecular
flexibility.
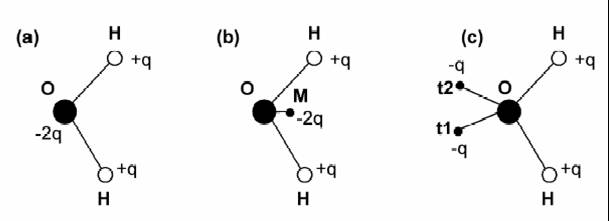
Fig 2 three kinds of water potential[7]
Flexible SPC Water parameters:
Charge on H: +0.41e and charge on O: -0.82e
Angle of HOH: 109.47°
Length of OH: 1.0Å
= 3.166Å
= 78.2 K
kOH = 4637 kJ/(mol Å2) kHOH = 383 kJ/(mol rad2)