


Next: Diffusion Data
Up: Results and Discussion
Previous: Results and Discussion
Figure 5:
Xe-Xe potential surface developed via Mathematica software. Nearest and next-nearest neighbor interactions where computed with a L-J potential where the yellow sphere represents a diffusing Xe atom.
|
|
We simulated the thermal diffusion of both Ar-Xe and Ar-Kr binary systems in 2 dimensions. The lattice was subject to a temperature gradient with a maximum temperature below the triple point. A Lennard-Jones potential was used in determining the potential energy surface or in general the energy barriers 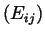 . Figure(1) depicts the a hypothetical potential energy surface in one dimension [6], and figure(5) displays a plot for Xe-Xe diffusion in 3D. The L-J potential of two atoms at a separation R is given as:
. Figure(1) depicts the a hypothetical potential energy surface in one dimension [6], and figure(5) displays a plot for Xe-Xe diffusion in 3D. The L-J potential of two atoms at a separation R is given as:
![\begin{displaymath}
U(R) = 4 \epsilon \left[\left(\frac{\sigma}{R}\right)^{12} - \left(\frac{\sigma}{R}\right)^{6} \right]
\end{displaymath}](img37.gif) |
(7) |
We assumed that the potential energy surface is independent of temperature such that the parameters
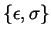 in the L-J potential between similar atoms can be taken from literature. With respect to the parameters
in the L-J potential between similar atoms can be taken from literature. With respect to the parameters
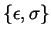 in the L-J potential between two different atoms,
in the L-J potential between two different atoms,
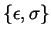 will be calculated with Lorentz-Berthelot mixing rules [5] [2]. Corrections due to excess fluctuations are not considered such that the equations below apply.
will be calculated with Lorentz-Berthelot mixing rules [5] [2]. Corrections due to excess fluctuations are not considered such that the equations below apply.
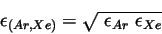 |
(8) |
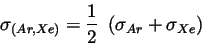 |
(9) |
Figures(6,7) display calculated barrier data for different lattice constants. The calculations were made for both a diffusing Ar and Xe atom surrounded by nearest and next-nearest Xe atoms (see figure(5)).
Figure 6:
Energy barriers calculated via L-J using Xe nearest and next-nearest neighbors with a lattice constant equal to 4.45
 . The diffusing atom is Ar in the red plot and Xe in the black plot.
. The diffusing atom is Ar in the red plot and Xe in the black plot.
|
|
As shown by the plots, the lattice constant is the most influential parameter in the calculations. In determination of an approximate lattice constant, we generated a lattice of 2 species in proportion to their concentration distributed randomly. This was then used to determine the total energy of the lattice (LJ interactions) as a function of lattice constant by performing the lattice sums. This function was then minimized to get the approximate lattice constant.
Figure 7:
A 2nd graph of the energy barriers calculated for Ar and Xe with a different lattice constant equal to 4.35
 .
.
|
|



Next: Diffusion Data
Up: Results and Discussion
Previous: Results and Discussion
Shawn A. Putnam
2001-12-17
![\includegraphics[height=.35\textheight, width=.75\textwidth]{LJSurface.bmp}](img35.gif)
![\includegraphics[height=.35\textheight, width=.75\textwidth]{LJSurface.bmp}](img35.gif)
![\begin{displaymath}
U(R) = 4 \epsilon \left[\left(\frac{\sigma}{R}\right)^{12} - \left(\frac{\sigma}{R}\right)^{6} \right]
\end{displaymath}](img37.gif)
![\includegraphics[height=.35\textheight, width=.75\textwidth]{EnergyBarrier4_45.bmp}](img41.gif)
![\includegraphics[height=.35\textheight, width=.75\textwidth]{EnergyBarrier4_35.bmp}](img43.gif)